The term stroke was originally referred to as “apoplexy”.
First written description was by Hippocrates over 2500 years ago.
The term “apoplexy” in Greek means “struck by violence”.
This signifies its sudden onset of symptoms, mainly paralysis, as being a sudden strike upon one’s physical wellness.
Brain Attack = Acute Stroke
Much of the knowledge gained about the disease process, its pathophysiology and clinical course in the last two decades.
There have been numerous advancements in ischemic stroke care not only with thrombolytics therapy, but also with endovascular therapy.
Both treatments have revolutionized the acute management of ischemic stroke.
Despite this, stroke remains a leading cause of disability worldwide.
This signifies the impact and burden stroke has on our society globally.
Timeline of Acute Ischemic Stroke Management
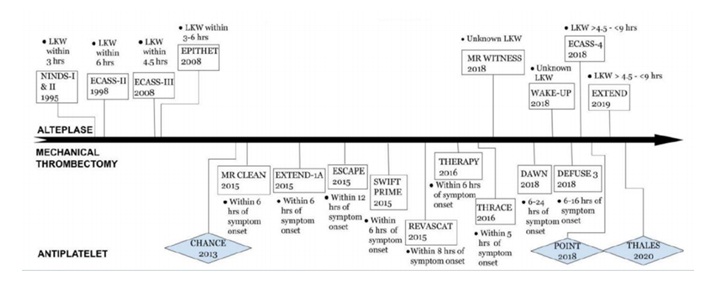
In this review I am going discuss the update in four main sub-headings.
1. Diagnostic evaluation of stroke etiology
1.1. General workup
The stroke workup is the set of diagnostic tests performed to gain insight into modifiable risk factors and stroke mechanism.
The stroke workup has fixed and variable components, the latter being contingent on clinical circumstances, initial testing, and therapeutic objectives.
Recent American Stroke Association guidelines on secondary stroke prevention include an algorithm for performing an evidence-based diagnostic evaluation.
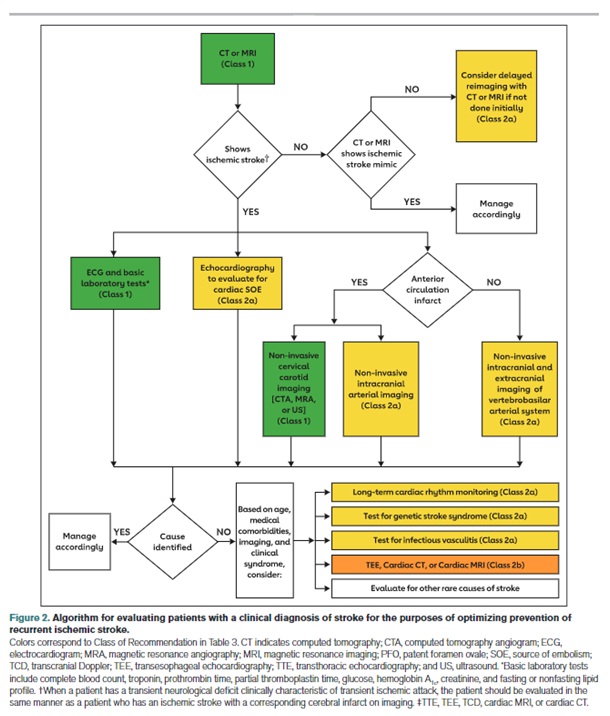
Fig 1– Algorithm for evaluating patients with a clinical diagnosis of stroke to optimize prevention of recurrent stroke
When a patient has a transient neurologic deficit clinically characteristic of transient ischemic attack (TIA), the patient should be evaluated in the same manner as a patient who has an ischemic stroke with a corresponding cerebral infarct on imaging.
Basic laboratory tests include complete blood count, troponin, prothrombin time, partial thromboplastin time, glucose, hemoglobin A1c, creatinine, and fasting or non-fasting lipid profile.
1.2 NIHSS
Every patient should have a National Institutes of Health Stroke Scale (NIHSS) assessment performed by a doctor or a nurse.
The acute NIHSS score, an excellent predictor of outcomes, also moderately correlates with acute diffusion-weighted imaging (DWI) and perfusion-weighted imaging lesion volumes on MRI.
NIHSS score of Zero does not mean that the patient has not had a stroke
Nearly 5% of strokes (mostly lacunar and infratentorial) will score a Zero on the NIHSS.
Zero-point strokes are not benign and have similar stroke recurrence rates as other stroke types.

Fig 2- How to recognize the signs of stroke 5
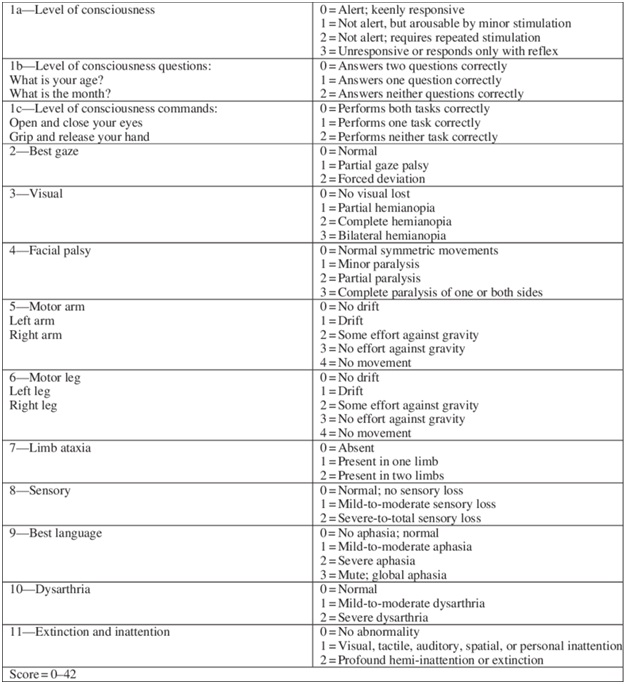
Fig 3-National Institutes of Health Stroke Scale (NIHSS)
1.3. Issue with posterior circulation ischemic stroke
Nearly 7% of acute ischemic strokes do not have a focal area of restricted diffusion on initial diffusion-weighted imaging
Patients with posterior circulation stroke are 5 times as likely to have diffusion-weighted imaging–negative stroke as patients with anterior circulation stroke.
The diagnostic yield of DWI falls with lower NIHSS scores but remains clinically meaningful, even for patients with resolved TIA.
MRI with DWI can detect acute ischemic stroke in about 20% of patients presenting with acute dizziness and vertigo.
The sensitivity of CT for diagnosing acute ischemic stroke within 24 hours after onset of the stroke symptoms (typically posterior inferior cerebellar artery infarcts) in this patient population is under 10%.
1.4. Cardioembolic Stroke
Long-term cardiac rhythm monitoring detects several fold more cases of atrial fibrillation than routine in patient monitoring following a stroke (12.1% versus 1.8%).
Oral anticoagulation is an AHA/ASA class 1 (level of evidence B-R) recommendation for atrial fibrillation, whether paroxysmal, persistent, or permanent.
Uncertainty remains around the optimal duration of monitoring.
The minimum burden of atrial fibrillation detection necessary to justify long-term oral anticoagulation remains unclear.
Transesophageal echocardiography (TEE) is generally considered more sensitive and specific than transthoracic echocardiogram (TTE) for detecting sources of cardioembolism.
But the discovery of pathologies that should alter management occurs in less than 5% of cases.
TEE may be less sensitive in detecting patent foramen ovale (PFO) than contrasted transthoracic echocardiography (TTE).
1.5 Embolic stroke of undermined source
To diagnose embolic stroke of undetermined source (ESUS), patients should have a stroke workup that includes, at a minimum, brain imaging, ECG, transthoracic echocardiography, cardiac monitoring for at least 24 hours, and imaging of both intracranial and extracranial arteries.
2. Intravenous Thrombolysis for Acute Ischemic Stroke
2.1. Core principles of IV Thrombolysis in acute ischemic stroke
- Viability of ischemic issue downstream from the occlusion is dependent upon the severity at infract core, penumbra, and state of oligemia as well as duration of the occlusion
- Reperfusion is the only effective strategy in acute ischemic stroke
- Irreversible ischemic brain injury occurs rapidly
- The efficacy of recanalization is highly time-sensitive
- The efficacy of IV rtPA is site specific (proximal, large volume clots are unlikely to recanalize)
2.2. Real world issue with rtPA
Extensive clinical trial data and “real world” experience support the efficacy and safety of recombinant tissue plasminogen activator (rtPA) as the primary treatment for acute ischemic stroke.
An improving deficit or low National Institutes of Health Stroke Scale score (NIHSS) does not exclude a patient from receiving rtPA if, after a careful neurologic history and examination, the stroke-related deficit would be disabling if it persisted without treatment.
While rtPA is approved for only up to 3 hours after symptom onset, data and guidelines support its use for up to 4.5 hours after symptom onset.
The use of factor Xa inhibitors is an increasing cause of exclusion from treatment with IV thrombolysis.
There are many gaps in the availability and use of IV thrombolysis and overcoming them is an important priority for improving stroke outcomes.
The sooner IV thrombolysis is delivered after symptom onset, the better the outcome
If treatment can be given by expedited emergency department care, two-thirds of patients will recover with no disability.
Advanced imaging reveals that the temporal progression of stroke is different in each patient; outcomes with treatment out to 9 hours after symptom onset in imaging-selected patients may result in comparable benefit to earlier treatment.
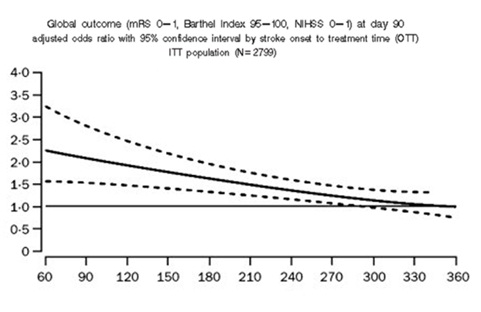
stroke onset to treatment time (OTT) {min}
Fig 4- Relation between time from symptom onset and treatment with recombinant tissue plasminogen activator (rtPA) and odds ratio of excellent outcome
2.3 Indications and Contraindications for rtPA (2019 update)

Fig 5– Indications and contraindications for rtPA 2019 update
IV thrombolysis can dissolve 20% to 30%of large vessel occlusion clots and should not be withheld in patients fulfill the treatment criteria except in rare circumstances.
Compared with rtPA (Alteplase), tenecteplase is more convenient, delivers the full dose faster, and produces comparable outcomes.
It is not yet approved by the FDA for IV thrombolysis.
2.4 IV Alteplase and Benefits versus Harm
Number needed to treat =3.1
Number needed to harm =33
> 10 patients helped for each patient harmed
2.5 IV Alteplase and Risk of Symptomatic Hemorrhage/Ham
Hemorrhage can be radiographically or clinically found
SITS-MOST criteria 2 parenchymal hemorrhage within 36 hours 3.7% vs. 0.6% (OR of 6.67)
Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007 Jan 27;369(9558):275-82. doi: 10.1016/S0140-6736(07)60149-4.
2.6. IV Tenecteplase
Why consider an alternative to IV Alteplase?
- Better (better reperfusion property)
- Safer (less hemorrhage)
- More convenient and given faster (easy to prepare and time saving)
Pharmacological differences between alteplase and tenecteplase 11
- Alteplase (tPA) is a recombinant version of naturally occurring enzyme called tissue plasminogen activator
- Tenecteplase (TNK) is a bioengineered variant of tPA to make it a better lytic by increasing its specificity to fibrin and more resistant to degradation by endogenous enzymes
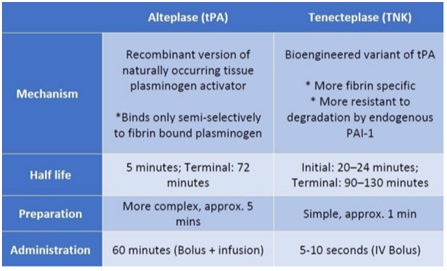
Fig 6-Differences between alteplase and tenecteplase
• TNK’s greater fibrin specificity which means fewer bleeding complications.
• TNK’s longer half-life may mean better clot lysis.
• TNK is easier to prepare and administer (a rapid, single-bolus) which may mean faster door-to-needle times and faster door-in-door-out times for transport (and hopefully translate into less disability after stroke).
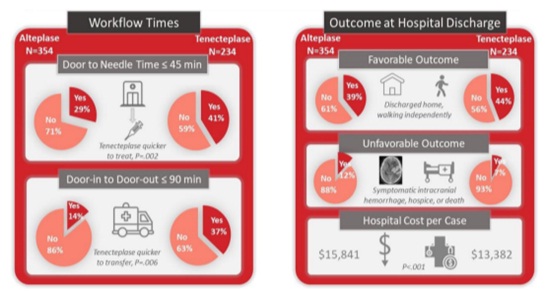
Fig.7 – Tenecteplase Versus Alteplase in Routine Clinical Practice
Warach SJ, et al, Prospective Observational Cohort Study of Tenecteplase Versus Alteplase in Routine Clinical Practice. Stroke. 2022 Dec;53(12):3583-3593. doi: 10.1161/STROKEAHA.122.038950
Evidence that Tenecteplase Is Noninferior to Alteplase for Acute Ischemic Stroke: Meta-Analysis of 5 Randomized Trials. Stroke. 2019; 50:2156-2162
Data Results Summary:
- Efficacy: TNK has consistently been shown to have equal or better rates of recanalization and reperfusion compared with tPA and TNK has consistently shown to have equal or better clinical outcomes compared with tPA
- Safety: TNK has consistently been shown to have equal or fewer intracranial hemorrhages compared with tPA. To date, there no reports of TNK-associated angioedema in the literature, but theoretically, this could occur
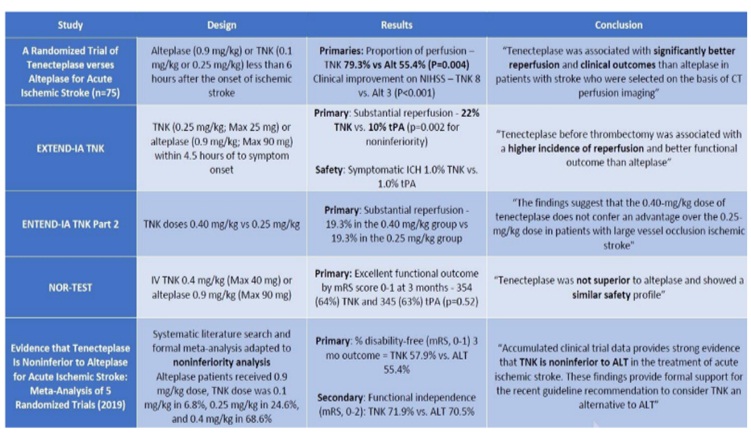
Fig 8- Clinical Trials of Tenecteplase Is Noninferior to Alteplase for Acute Ischemic Stroke
Evidence that Tenecteplase Is Noninferior to Alteplase for Acute Ischemic Stroke: Meta-Analysis of 5 Randomized Trials. Stroke. 2019; 50:2156-2162
Benefits of using tenecteplase
- Well-characterized mechanism of action
- Eligibility for TNK is identical to the eligibility criteria for tPA with Alteplase
- Reversal of bleeding complications with TNK is identical to Alteplase reversal
- The cost of TNK is currently less than tPA
- TNK requires less pharmacist/nursing time as it is easier and faster to prepare and administer
- 25 mg/kg is statistically non-inferior overall and improve with Large Vessel Occlusion (LVO).
3. Mechanical Thrombectomy for Acute Ischemic Stroke
3.1. General Views
Endovascular therapy (EVT) has become the consensus approach for appropriately selected patients and can produce dramatic improvements in clinical outcomes for patients with large vessel occlusion (LVO).
The positive EVT trials of 2015 established EVT as clearly beneficial compared to medical management.
In these later trials, the number needed to treat (a measure of the effect size of the intervention) to change a patient’s clinical course from moderate-or-severe disability to functional independence ranged from 3 to 5.
In large, real-world observational cohorts, rates of good neurologic outcomes are remarkably similar to those of the randomized clinical trials (approximately 55% functional independence at 90 days), relative to the control populations of the clinical trials who did not receive EVT (26.5%).
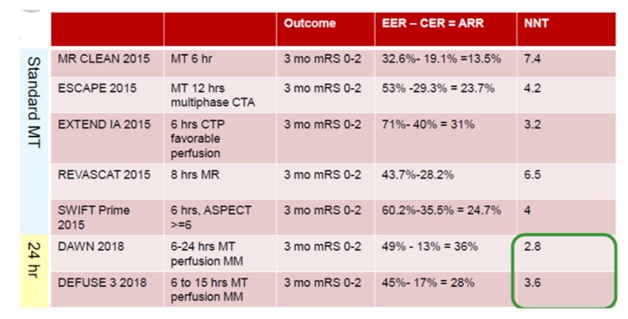
Fig 9-Number need to treat NNT –Acute ischemic stroke treatment with Thrombectomy
3.2. Wake up stroke. What can we do?

Thomalla G, Simonsen CZ, et al, WAKE-UP Investigators. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N Engl J Med. 2018 Aug 16;379(7):611-622. doi: 10.1056/NEJMoa1804355
- MRI DWI-positive FLAIR-negative
- Present within 4.5 hours of symptoms recognition
- 89% had wake-up stroke
- Excluded patients thrombectomy was planned
- Randomized to 0.9 mg/kg rtPA vs. placebo
- A favorable outcome at 90 days was reported in 131 of 246 patients (53.3%) in the alteplase group and in 102 of 244 patients (41.8%) in the placebo group (adjusted odds ratio, 1.61; 95% confidence interval [CI], 1.09 to 2.36; P=0.02).
- The median score on the modified Rankin scale at 90 days was 1 in the alteplase group and 2 in the placebo group (adjusted common odds ratio, 1.62; 95% CI, 1.17 to 2.23; P=0.003).
- There were 10 deaths (4.1%) in the alteplase group and 3 (1.2%) in the placebo group (odds ratio, 3.38; 95% CI, 0.92 to 12.52; P=0.07).
- The rate of symptomatic intracranial hemorrhage was 2.0% in the alteplase group and 0.4% in the placebo group (odds ratio, 4.95; 95% CI, 0.57 to 42.87; P=0.15).
Conclusions:
• In patients with acute stroke with an unknown time of onset, intravenous alteplase guided by a mismatch between diffusion-weighted imaging and FLAIR in the region of ischemia resulted in a significantly better functional outcome and numerically more intracranial hemorrhages than placebo at 90 days
3.3. Direct mechanical thrombectomy (MT) vs. bridging IV rtPA +MT
3.3.1. Earlier Trials of Direct mechanical thrombectomy (MT) vs. bridging IV rtPA +MT

early recanalization (ER) (ER; ≤3 hours after start of IV-tPA)
meta-analyses, 26 studies, 2063 patients
overall incidence of partial or complete ER was 33% (95% CI, 27–40), varied according to occlusion site:
- 35% (complete ER 21%) for proximal MCA
- 13% (complete ER 4%) for ICA
- 13% (complete ER 4%) for basilar occlusion
proximal occlusion and higher NIHSS were the most consistent no-ER predictors
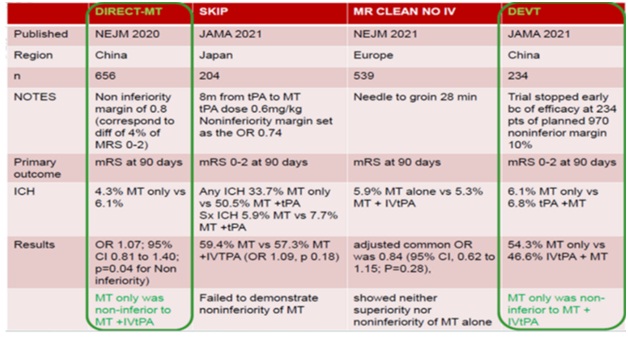
Fig 10- Two failed trials of Direct mechanical thrombectomy (MT) vs. bridging IV rtPA +MT
3.3.2. New Trials of Direct mechanical thrombectomy (MT) vs. bridging IV rtPA +MT
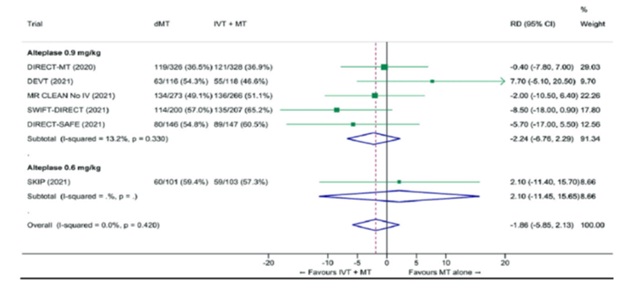
Fig 11-Two failed trials of Direct mechanical thrombectomy (MT) vs. bridging IV rtPA +MT
(a) DIRECT-SAFE trial
Mitchell PJ et al, DIRECT-SAFE: A Randomized Controlled Trial of DIRECT Endovascular Clot Retrieval versus Standard Bridging Therapy. J Stroke. 2022 Jan;24(1):57-64. doi: 10.5853/jos.2021.03475.
(b) SWIFT DIRECT trial
Prof Urs Fische et al, “Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: an open-label, blinded-outcome, randomised non-inferiority trial” The Lancet Volume 400, Issue 10346, p104-115, July 09, 2022, July 09, 2022, doi: 10.1016/S0140-6736(22)00537-2
- DIRECT-SAFE and SWIFT-DIRECT show that IV alteplase should not be withheld when endovascular thrombectomy is being done
- MT was not shown to be non-inferior to IVtPA+MT in both studies
3.4. Basilar artery occlusion (BAO)
3.4.1. Outcome of BAO in general

Data were available on 82 patients
The case fatality was 40%.
Among survivors, 65% remained dependent (Rankin score 4–5).
Patients younger than 60 years (odds ratio = 3.1 (95% confidence interval, 1.0 to 9.5)) and those with a minor stroke (OR = 3.1 (1.0 to 9.6)) were more likely to have a good outcome (Rankin score 0–3).
Patients with a progressive stroke were less likely to have a good outcome (OR = 0.3 (0.08 to 1.2)) than patients with a maximum deficit at onset or fluctuating symptoms at presentation. 19
Conclusions: Conventional treatment of symptomatic basilar artery occlusion is associated with a poor outcome in almost 80% of patients, which emphasizes the importance of the search for a more effective treatment approach.
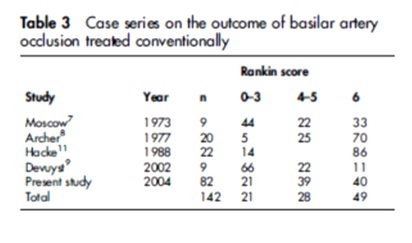
Fig 12– Outcome of BAO treated conventionally
3.4.2. Basilar artery occlusion: randomized control trials
(a) Two negative trials
2 randomized trials comparing MT vs medical management BASICS (Basilar Artery International Cooperation Study- 6 hours) and BEST trial (Basilar Artery Occlusion Endovascular Intervention Versus Standard Medical Treatment- 8 hours)
Both of demonstrated equivocal benefit between the two modalities
BA occlusion trials used primary outcome mRS 0-3 at 90 days
No difference in outcomes
Both trials showed no difference in outcomes with MT compared to medical treatment
Van der Hoeven EJ, et al; BASICS Trial. The Basilar Artery International Cooperation Study (BASICS): study protocol for a randomised controlled trial. Trials. 2013 Jul 8;14:200. doi: 10.1186/1745-6215-14-200. 20
Liu X, et al; BEST Trial. Acute basilar artery occlusion: Endovascular Interventions versus Standard Medical Treatment (BEST) Trial-Design and protocol for a randomized, controlled, multicenter study. Int J Stroke. 2017 Oct;12(7):779-785. doi: 10.1177/1747493017701153.
(b) Two positive studies
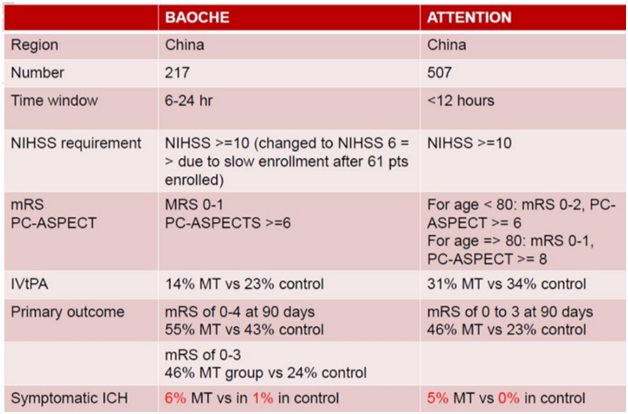
Tao C, et al. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med. 2022; 387:1361–1372. doi:10.1056/nejmoa220631 (BAOCHE)
Jovin TG, et al. Trial of thrombectomy 6 to 24 Hours after stroke due to basilar-artery occlusion. N Engl J Med. 2022; 387:1373–1384. doi:10.1056/nejmoa2207576 (ATTENTION)
4. Antiplatelets Update 21
4.1. Summary of Antiplatelet Agents
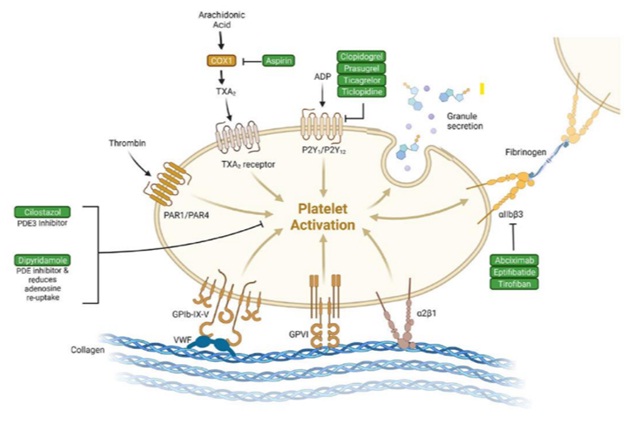
Fig 13-Antiplatelet agents and their mechanisms of action on the platelet
Antiplatelet agents inhibit platelet aggregation and reduce the risk of acute ischemic stroke (AIS) or TIA.2
Aspirin, clopidogrel, dipyridamole/ aspirin, cilostazol ticagrelor and prasugrel are commonly used antiplatelet agents.
Due to the complexity of stroke etiology and diverse mechanisms of antiplatelet agents (figure 13), it is essential to select optimal antiplatelet therapy in the real-world practice.
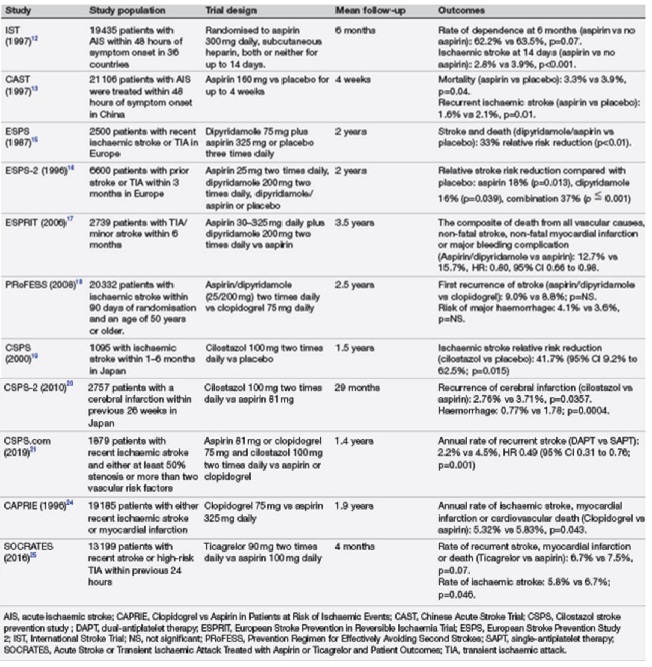
Fig 14– Landmark randomized controlled trials evaluating antiplatelet therapy in secondary stroke prevention
4.2. Newer antiplatelet agents
(a) Prasugrel
- There is not enough evidence to support the use of prasugrel instead of using clopidogrel in individuals with a history of ischemic stroke or TIA.
(b) Ticagrelor
- Ticagrelor is found to be associated with increased risk of major bleeding events, bradycardia and increased dyspnea when compared with other antiplatelet agents.
- No head-to-head trials with clopidogrel either alone or I combination with aspirin have been undertaken.
(c) The glycoprotein IIb/IIIa inhibitors (GP2b3ais)
They have been evaluated for use in the acute period after ischemic stroke.
Mechanism of action is by inhibiting the platelet cell surface glycoprotein IIb/IIIa receptor that stops platelet aggregation by inhibiting fibrinogen molecule binding.
In general, the drugs are short-acting and administered intravenously, making them less likable for long-term use.
4.3. Some clinical pearls for use of antiplatelets
(a) Stroke While on Antiplatelet Therapy
- No antiplatelet agent was 100% effective in preventing recurrent cerebrovascular events in the clinical trials.
- A meta-analysis of observational studies found evidence in favor of switching to an entirely new antiplatelet combination after a recurrent event, with a reduced incidence of cardiovascular events on follow-up, there are no randomized controlled trials to guide clinicians.

Lee M, Saver JL, Hong KS, Rao NM, Wu YL, Ovbiagele B. “Antiplatelet regimen for patients with breakthrough strokes while on aspirin: a systematic review and meta-analysis”. Stroke. 2017;48(9):2610-2613. doi:10.1161/ STROKEAHA.117.017895
(b) A few practical points in cases of recurrent stroke while on antiplatelet therapy
- It is vital to ensure that the patient is adherent with the antiplatelet as prescribed.
- Concurrent medication review may reveal interacting drugs that need to be removed.
- When there is an antiplatelet treatment failure, search for cardioembolic causes of stroke that could respond to anticoagulants.
- Optimize secondary prevention (rather than switching antiplatelets) such as increasing the dose of statin, improving blood pressure and/or blood glucose control and lifestyle modifications etc.
(c) Consideration of Dual Antiplatelet Therapy (DAPT)
- The current consensus is that DAPT is appropriate in the acute phase (defined as the first 30 days poststroke) postischemic stroke, especially when initiated promptly, but there appears to be no benefit in continuing this further.
- Commence DAPT with aspirin plus clopidogrel within 24 hours of minor stroke (defined as NIHSS ≤3) or high-risk TIA (defined as ABCD2 ≥ 4,) and continuing for 21 days.
- CHANCE and POINT trials
(d) Using Antiplatelets and Anticoagulant in Combination
- When generalizing to the ischemic stroke population, there appears to be little benefit of combination therapy.
- In certain situations when a patient presents with stroke and unstable coronary artery disease, the use of combinations of antiplatelets and anticoagulant drugs may provide extra benefit.
- Currently, there is no RCT level evidence of the benefits of combination therapy poststroke assessing this particular group.
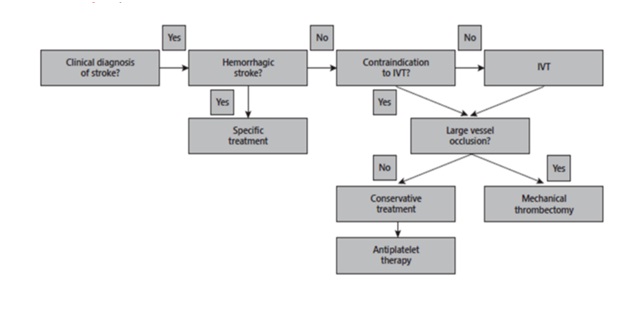
5. Summary Flowchart
5.1. Acute ischemic stroke treatment Flowchart. (IVT, intravenous thrombolysis)
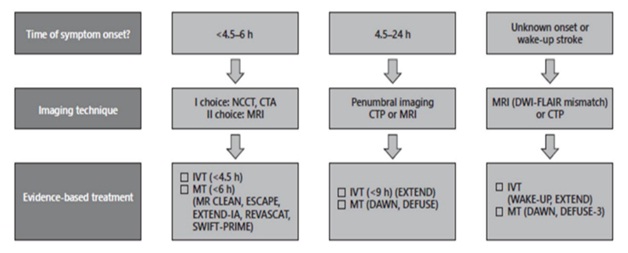
5.2. Flowchart for neuroimaging-guided acute ischemic stroke treatment
(NCCT, noncontrast cerebral tomography; MRI, magnetic resonance imaging; CTP, cerebral tomography perfusion; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; IVT, intravenous thrombolysis; MT, mechanical thrombectomy)
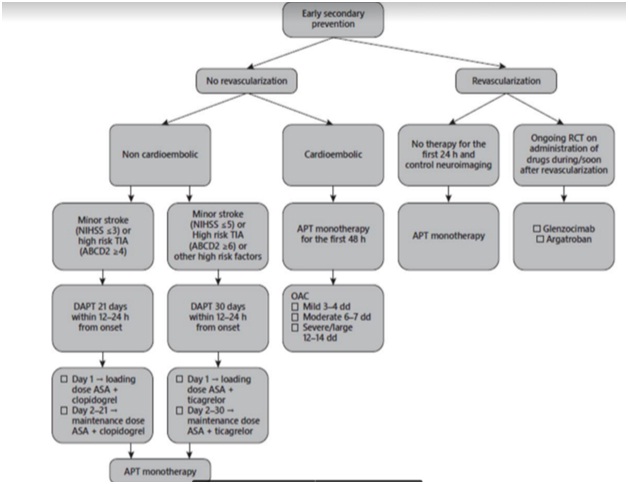
5.3. Flowchart for early secondary prevention in acute ischemic stroke
TIA, transient ischemic attack; DAPT, dual antiplatelet therapy; ASA, aspirin; APT, antiplatelet therapy; OAC, oral anticoagulation; RCT, randomized controlled trials
Author Information
Dr. Thar Thar Oo
Senior Consultant Neurologist, Director (Clinical Neurology)
Neurologist-in-Charge (Stroke Unit, Asia Royal Hospital, Yangon, Myanmar)







