Management Of Gout

1 Definition
Inflammatory arthritis triggered by crystallization of monosodium urate within the joints when uric acid, the end-product of purine metabolism, exceeds its limit of solubility in body fluids.
Hyperuricaemia is NOT gout, rather it is a risk factor for gout.
(Limit of urate solubility at physiologic temperature and pH is 6.8mg/dL, approx 404 umol/L)
Note: Gout Classification Criteria by American College of Rheumatology/ European League Against Rheumatism (2015) can be assessed online at: ARTHRITIS & RHEUMATOLOGY Vol. 67, No. 10, October 2015, pp 2557–2568.
2 Epidemiology
- Global prevalence – 0.08%
- 1-2% of adult men in western countries
- Ratio of male to female: 3 or 4 to 1
- Peak onset of gout in males 40-50 years and in female is after 60 years
3 Pathophysiology
- Factors for Increased Urate Level
- Overproduction
- Under-excretion – believed to be the major factor
- Possibly – Increased Intake
- The crystals commonly deposit in tissues with limited blood flow – tendons, cartilage, ligaments, bursa, skin in areas that are cooler or around distal joints.
- Tophi are large urate deposits in skin
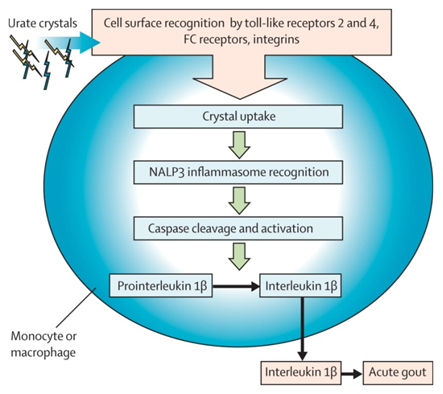
4 Clinical Features
Stages of Development
- Asymptomatic hyperuricemia
- Acute gouty arthritis
- Inter-critical (interval) gout
- Chronic tophaceous gout
Acute and chronic gout will be discussed further.
4.1 Acute Gout
History
- Abrupt onset, usually at night, severe excruciating pain/ redness
- Usually at the extremity or distal joints
- Usually mono-arthritis (80%)
- Can be associated with triggering factors such as surgery, infection
Examination
- Swollen, red, tender joint, inflamed adjacent area
4.2 Chronic Gout (+/- Tophi)
- Recurrent joint pain / inflammation in a polyarticular pattern
- Tophi formation / Joint destruction
- Can mimic inflammatory arthritis eg RA
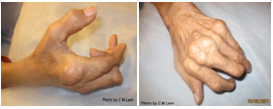
Fig 2: Images of chronic tophaceous gout minicking RA
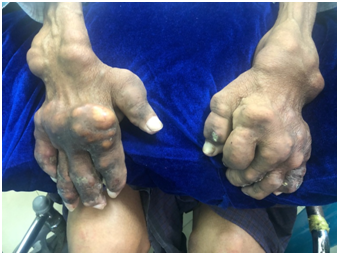
Fig 3: Images of disabling chronic tophaceous gout
5 Investigations
- Full blood count
- Neutrophil leucocytosis can be seen in acute attack, should be differentiated from septic arthritis
- Serum uric acid level
- Usually raised, can be normal or even low in acute attack
- Serum creatinine
- To exclude CKD that can lead to hyperuricemia and gout
- Investigations for detection of associated cardiovascular risk factors
- Lipid profile
- Blood sugar
- ECG
- X ray of the affected joint – cortical break in bone suggestive of gout (overhanging edge with sclerotic margin), can be normal in acute gout
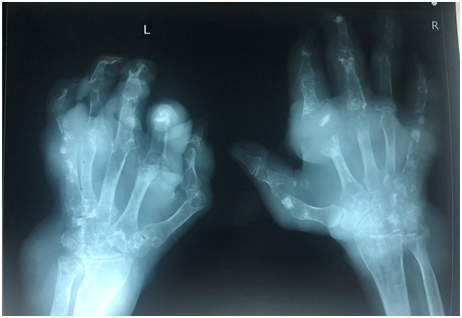
Fig 4: Images of an x ray on hands of severe chronic tophaceous gout
- Ultrasound examination of the affected joint – (if resources permit) – hyperechoic band over anechoic cartilage (double contour sign), presence of tophi, joint effusions and erosions
- Polarized light microscopy of joint aspirate – (if resources permit) – needle shaped, negatively birefringent gout crystals
6 Treatment
6.1 Acute Gout
- Aim: to suppress the inflammation
6.1.1 Pharmacological treatment: –
- Non-steroidal anti-inflammatory drugs (NSAIDS)
- Start early, may need to keep until 48 hours after the resolution of attack
- Beware of contraindications Eg Peptic ulcer disease, renal impairment
- Use proton pump inhibitors if necessary
- Any agent can be used
- Give full dose
- Colchicine
- For acute gout and prophylaxis of flares
- Be cautious in renal, hepatic and bone marrow disease
- 1.2 or 1 mg stat followed by 0.6 or 0.5 mg 1 hour and 12 hours later and then twice a day (or once a day) until acute attack resolved
- Dose reduction in renal failure:
- According to Creatinine clearance (Cr. Cl)
- 30 – 50 ml/min: 0.5 mg maximum daily
- 10 – 30ml/min: 0.5 mg 2 – 3 X per week.
- To avoid if CrCl < 10 ml/min
- Drug interactions – statins, fibrates, clarithromycin, erythromycin, ketoconazole, itraconazole, verapamil, diltiazam, protease inhibitors, cyclosporine,
- Corticosteroids
- Indicated if contraindication to
- NSAIDS: Peptic Ulcer Disease, renal, liver and cardiac failure
- Colchicine: Renal (CCT < 50 ml/min), liver dysfunction
- Not effective with NSAIDS
- Steroids can be used in the form of
- Intra-articular – limited to one or two joints, amenable to aspiration and in the absence of joint sepsis – refer to specialist
- Systemic (Prednisolone 15 – 30 mg or equivalent dose of injectable form / day for 5 days)
- IM ACTH 1mg
- Indicated if contraindication to
- IL-1 inhibitors (Canakimumab, Rilonacept, Anakinra) – currently unavailable in Myanmar
6.1.2 Supportive management
- Rest the joint / ice the joint
6.2 Chronic Gout – Urate Lowering Therapy (ULT)
6.2.1 General Information
- Should be initiated concurrently with acute gout treatment of 2-4 weeks after flare resolution or simultaneously; consider patient factors
- Target serum uric acid level – 6mg/dl (350μmol /L), 5 mg/dl (300μmol /L) in association with CKD, uric acid stone
- Indications
- Two or more acute attacks in a year
- Single attack with
- Tophi in soft tissues or subchondral bone
- Clinical or radiographic signs of chronic gouty arthritis
- Renal insufficiency
- Nephrolithiasis
- Very high serum uric acid at presentation (>9mg/dL)
- Lifelong treatment
6.2.2 Medications
- 3 main classes
6.2.2.1 Xanthine Oxidase Inhibitors – Allopurinol, Febuxostat
- Allopurinol –
- First Line
- Renally excreted, usually safe
- S/E – GI,rash, sarcoid like reaction, Allopurinol Hypersensitivity Syndrome (AHS)
- Drug interaction – esp. with 6 Mercaptopurine, Azathioprine, warfarin, theophylline.
- Dose increase from 100 mg to 800 mg/day (usually 300mg/day)
- Dose reduction in renal impairment
- To increase allopurinol slowly – every 2-4 weeks, monitor for adverse effects.
- Febuxostat
- Non-purine analog
- Metabolized in the liver
- Dose: 40mg -120mg
- Contraindicated for use with Azathioprine, Mercaptopurine, Theophylline
- No cross reactivity with Allopurinol – used in Allopurinol hypersensitive patients (with caution)
6.2.2.2 Uricosuric agents – Probenecid, Sulphipyrazone, Benzbomarone,
- Probenecid (500 mg – 2g/ day in 2 divided dose)
- Sulphinpyrazone (50 mg BD to 100 – 200 mg 3 – 4x per day)
- Benzbromarone (50 – 200 mg/day) – contraindicated in liver disease – fatal hepatitis
- All are relatively ineffective compared to xanthine oxidase inhibitors.
- Not used in:
- renal calculi
- Renal insufficiency (24 hrs urinary CrCl < 50 ml/min) except Benzbromarone (can be use when 24 hour urinary Cr Cl > 20 ml/min)
- Combination therapies with xanthine oxidase inhibiors
6.2.2.3 Urate oxidase –
Uricase –Pegloticase – currently unavailable in Myanmar
6.2.3 Changing ULT – to consider when
- High serum uric concentrations (>6 mg/dl) despite maximum or maximum-tolerated dose
- Continued frequent gout flares (>2 flares/year)
- Non-resolving subcutaneous tophi.
6.2.4 Prophylaxis for acute attacks when initiating urate lowering therapy
- Acute fall in serum uric acid can precipitate gouty flare
- Reduces rate of recurrent attacks whether or not the uric acid is normal.
- Duration – 3 – 6 months since the start of urate lowering therapy
- Longer duration if large / multiple tophi
- Medications
- Low dose colchicine 500 – 1000 mcg /day
- Low dose NSAIDs
- Low dose steroid
- Choice – depends on patient factors
Fig 5: Targets for intervention in the treatment and prophylaxis of gout
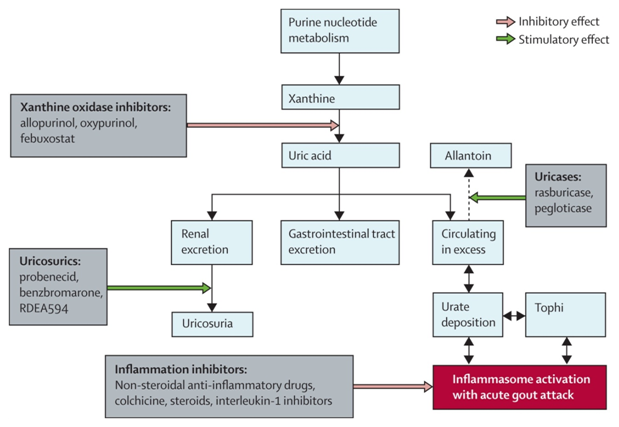
7 Lifestyle and Adjunctive Therapies
- Obesity
- Risk of gout significantly higher in men with BMI >25.
- Mean weight loss of 5 kg resulted in a mean SU lowering of 1.1 mg/ dl
- Alcohol abuse
- Drinking 2 or more cans of beers per day increase risk of gout by 2.5X
- A unit of beer raised SU concentrations by 0.16 mg/dl.
- Low purine diet
- Strict dieting can only lower serum uric acid by at most 120 mol/L (2 mg/dl)
- Moderation in dietary purine consumption is indicated in those who habitually eat large amount of purine-containing food – red meat, seafood, organ meat
- Plant based foods – no significant hyperuricemia (myths – to avoid plant-based foods – a hand-span above and below the ground, vegetables rich in seeds such as tomato. There is no evidence on worsening the hyperuricemia by ingesting plant-based foods)
- Fructose – Ingestion of 1 gm of fructose/kg of body weight increases SU concentration by 1–2 mg/dl within 2 hours of ingestion; avoid soft drinks
8 Comorbid Conditions
- Hypertension
- Diuretics – consider changing to other drugs
- losartan – has uricosuric effect – to consider to use it
- Hyperlipidaemia
- Fenofibrate has uricosuric effect – but not recommend switching from current treatment
- Low dose aspirin – can continue if there are indications, even though it can lead to hyperuricemia
9 When to refer to Physician/ Rheumatologist
- Unable to achieve the target uric acid level with optimal dosing
- Severe comorbidities
- Frequent gout flares despite regular treatment
- Severe complications from drug treatment
References
- 2020 American College of Rheumatology Guideline for the Management of Gout; Arthritis Care & Research Vol. 0, No. 0, June 2020, pp 1–17
DOI 10.1002/acr.24180 - The British Society for Rheumatology Guideline for the Management of Gout, Rheumatology, Volume 56, Issue 7, July 2017, Pages e1–e20,
- Gout, Hyperuricemia, and Crystal- Associated Disease Network Consensus Statement Regarding Labels and Definitions for Disease Elements in Gout; Arthritis Care & Research Vol. 71, No. 3, March 2019, pp 427–434, DOI 10.1002/acr.23607
- UpToDate ;https://www.uptodate.com/contents/search
Author Information
Professor Cho Mar Lwin
Consultant Physician and Rheumatologist




